Amazing! Scientists discover a new function of zeolite: it has drug-removing properties
Zeolites belong to the class of aluminosilicate microporous solids with strong and varied catalytic activities, which makes them suitable for almost all types of industrial processes, especially due to their environmentally friendly properties. Another important feature of zeolites is their enormous adsorption capacity.
Therefore, it goes without saying that the widespread use of zeolites in environmental protection is mainly based on the adsorption capacity of substances that may be harmful to the environment, such as drugs, pesticides, or other industrial pollutants. On the other hand, zeolites are also considered as drug delivery system (DDS) carriers for many pharmacologically active agents.
The enhanced bioactivity capabilities of DDS zeolites as drug-loaded nanoplatforms were demonstrated, making the system more specific and efficient than the drug itself. Indeed, these two applications of zeolites illustrate the importance of (non-)reversibility of the adsorption process.
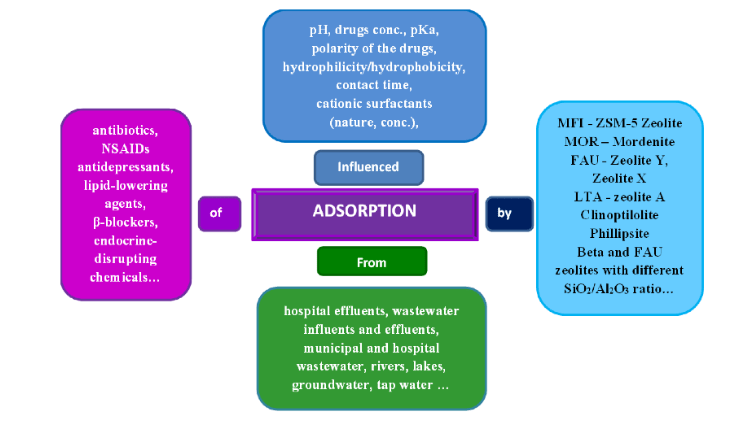
Among numerous attempts, the use of natural zeolites to purify water from pollutants has been highlighted as a simple, efficient, low-cost, and environmentally friendly procedure. Natural Zeolites (whether in raw or modified form) are well-known materials for adsorbing pesticides from wastewater. First, studies usually start with neat structures to elucidate key parameters such as Si/Al ratio, surface area, and extra-framework ions.
The experimental design relies on loading optimization; however, a practical approach is to use low loads to increase adsorption capacity, which is one of the function of zeolite. In order to participate in this exploration, researchers sometimes report loadings beyond the initial adsorption amount many times, although this has no physical significance. Another challenge is to detect important parameters for the efficient removal of ambient significant concentrations, which are typically used for drugs at the ng/mL level.
Such low concentrations mask the true sorbent performance, and laboratory studies often use mg/L concentrations to increase experimental sensitivity. Searching the literature can reveal published results testing a large number of zeolite structures with the aim of testing their ability to remove drugs, i.e. their adsorption from wastewater.
New Function of Zeolites
The function of zeolite is more. Zeolites sometimes require functionalization/compound preparation to establish new and improved properties. For example, MFI zeolites have the disadvantage of low adsorption capacity, combined with excellent adsorbents, the removal technology can be extended from adsorption to catalytic degradation in the presence of suitable oxidants.
For example, a composite prepared from MFI zeolite and carbon adsorbent was proposed for the adsorption of ciprofloxacin at 150 ppm. A positive example of the use of zeolite itself in drug therapy is clinoptilolite, which can stand out as a potent antidote, antioxidant and anti-inflammatory agent. Zeolites are currently considered dietary supermaterials, which is sometimes an overstatement. Every drugstore is selling zeolite-based nutritional formulas with added vitamins, enzymes, etc., often without any scientific evidence to back up claims of high nutritional value.
Antioxidant, antibacterial, detoxifying, and anticancer activities are often attributed to synthetic and natural zeolites. However, a rigorous survey of the existing literature has yielded some controversial findings. For example, the ability of zeolites to trap free radical species so that they can be safely removed from the body is the cornerstone of much research.
Part of this is actually true, the zeolite structure does remove free radical species, although they should not be part of the human diet and it was used in cattle, as they bind tightly in the gastrointestinal tract to mycotoxins in animal feed, thereby reducing their bioavailability.
